CHEM-E-CARS OF REACTICS
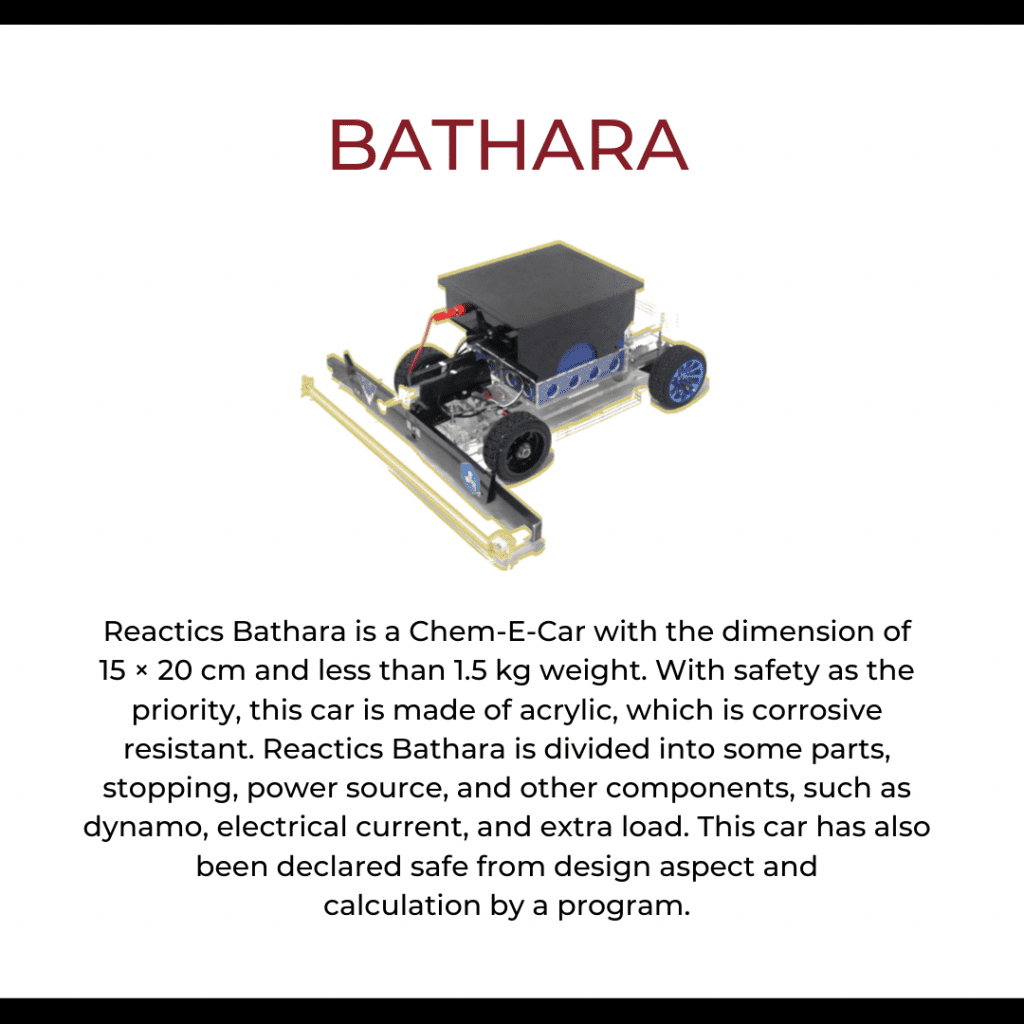
Reactics Bathara
Click to view more information
Flimo
Click to View More Information
Sembrani 1.0
Click to View More Information
Gatotkaca 1.0
Click to view more information
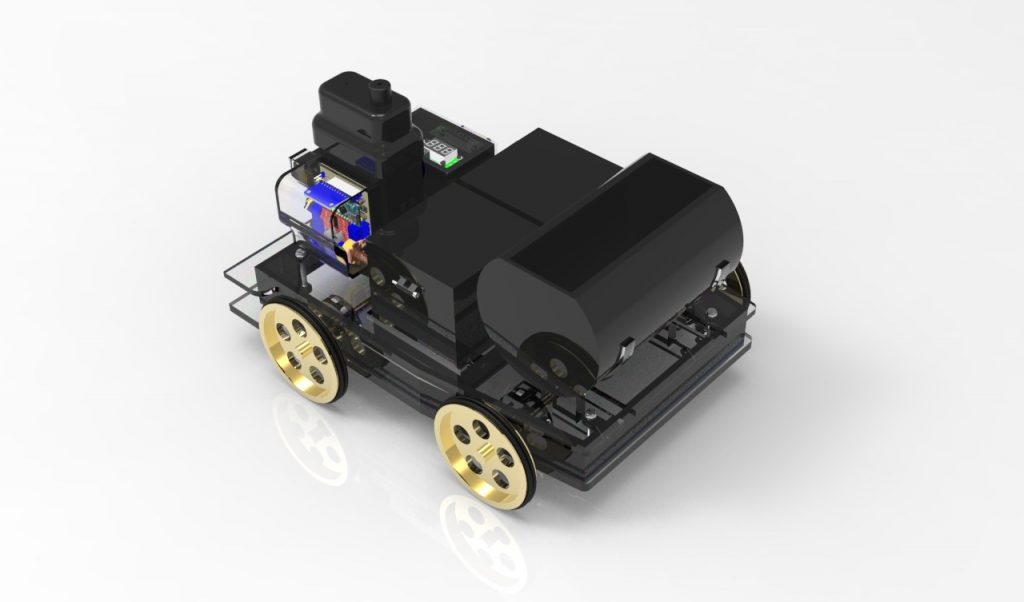










Reactics Abiyasa
Reactics Abiyasa is a team that focuses on designing a pressurized gas car. This car has the dimension of 20 cm x 30 cm and is equipped with a pneumatic drive system. The main power source for this car is the decomposition reaction of hydrogen peroxide (H202) which produces pressurized air supplied to the pneumatics. The pneumatic drive system then transforms the energy from pressurized air into linear motion, and with the help of a crankshaft, converts the linear motion into rotational motion.

Reactics Abimanyu
Reactics Abimanyu is made of corrosion-resistant acrylics that have light but durable characteristics for the base of the car and the battery container. While the load pack is made of acrylics. From front to back, the body parts can be divided into four parts: power source, additional load, stopping mechanism, and miscellaneous components such as DC motor, electric circuit, and solenoid push. The electric circuit consists of a light-dependent resistor, magnetic stirrer, solenoid push, and microcontroller for stopping mechanism control. To ensure that Reactics Abimanyu will stop accurately at a specific distance, the motor has been chosen proportionally with
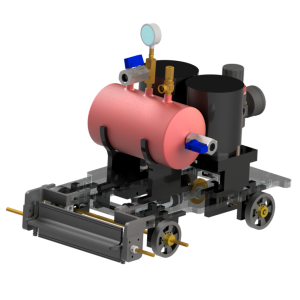
Reactics Rahwana
Reactics Rahwana is a powerful pressurized gas car with 32.6 cm length x 21 cm wide x 22.6 cm height dimension powered by using a decomposition reaction of Hydrogen Peroxide 10% within the vessel catalyzed by KI. This car has a minimalist design to reduce the charges. The car has good corrosion resistance and is secure in the operation due to its safety valve used to maintain the pressure inside the vessel which is made from stainless steel 304. This car uses pneumatic for transforming high pressurized gas to kinetic energy.

Reactics Janaka
Reactics Janaka is a chem-e-car that applies the concept of voltaic cell for its power sources to move the car and gas producing reaction to stop the car. It has a dimension 38 cm x 28 cm and when disassembled has a height less than 20 cm. The power source of Reactics Janaka uses aluminium-air battery that consist of aluminium foil as anode, activated carbon as O2 adsorbent and cathode, NaCl 1 M as electrolyte, filter paper as separator, and copper plate as current collector

Reactics Palapa
Reactics Palapa is a chem-e-car that applies the concept of voltaic cell for its power source and uses the Chameleon reaction as its stopping mechanism. The power source consists of 12 aluminum-air cells in series, providing specific current and voltage needed to run the car. Meanwhile, the car will automatically stop by a colour change reaction. Reactics Palapa has a dimension of 35 cm x 25 cm, with empty weight 1,51 kg.

Reactics Indra
Reactics Indra is a powerful pressurized gas car which is powered by high pressure oxygen gas. Oxygen gas is produced by Hydrogen Peroxide decomposition reaction inside a stainless steel pressure vessel. High pressure oxygen flows through a polyurethane hose into a water tank and pushes the water through another hose into the car turbine box. The high pressure water from the water tank will rotate the turbine. This rotation from the turbine will be connected into the shaft by pulley with 5:1 ratio and belt. The dimension of Reactics Indra is 33.5 cmx 26cm x 31.9cm with 4 wheels under the acrylic chassis. Reactics Indra used various materials such as acrylic, PLA Plastic, stainless steel, brass, and many other materials.
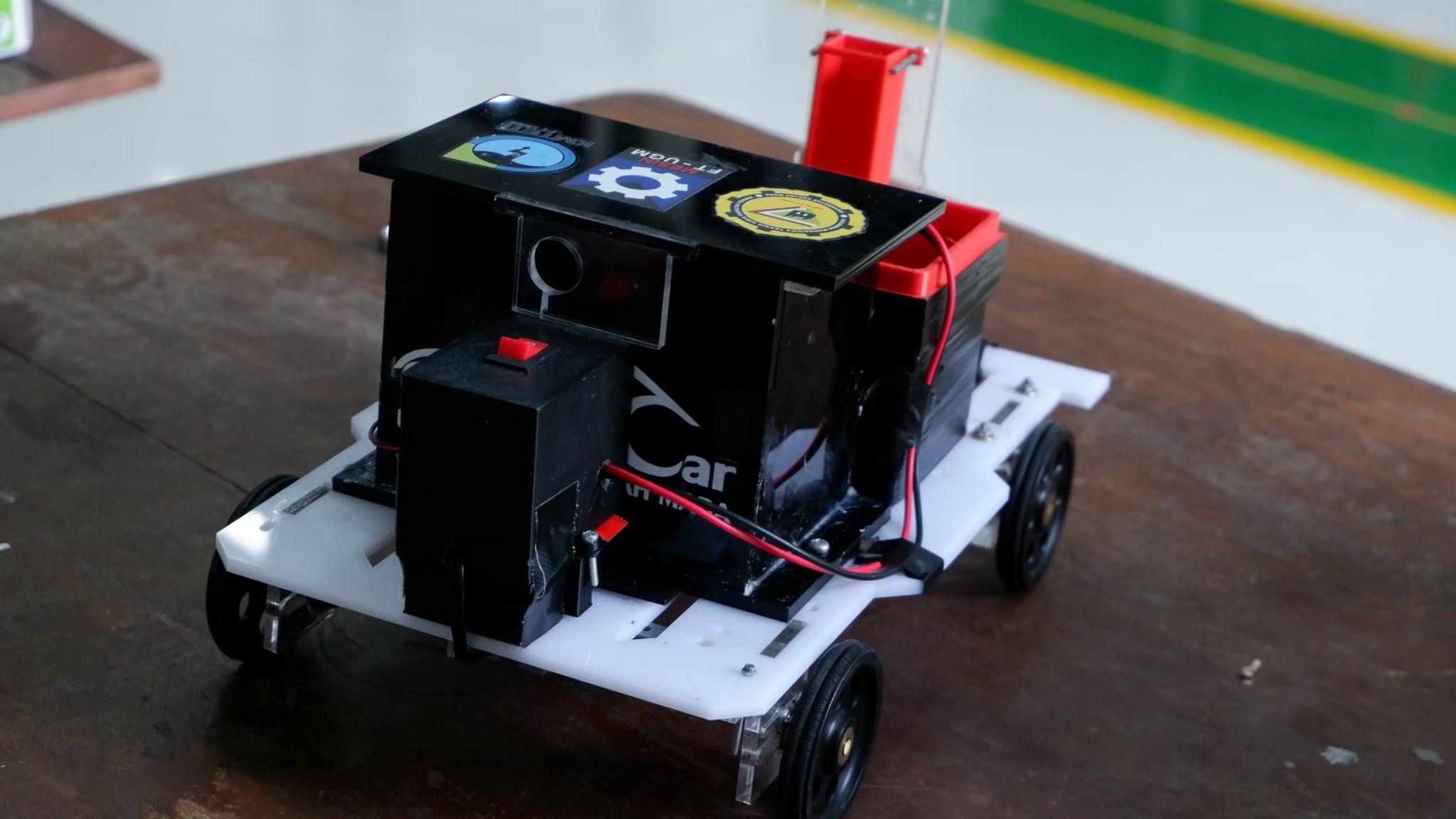
Reactics Anoman
Reactics Anoman is a Chem-E-Car that uses the voltaic cell reaction for its power source and uses a reaction that produces carbon dioxide gas as its stopping mechanism. The dimensions of this car are 295 mm c 210 mm x 100 mm. The body of the car mostly uses acrylic material as well as 3D printer filaments. Reactics Anoman uses a reaction of zinc metal and air, with NaOH as the electrolyte. This reaction will produce a voltage of 1.45 volts per cell. Most of the materials used in the reaction are classified as safe and not harmful for the environment, therefore this car has an added value in the safety aspect.

Reactics Bisma
Reactics Bisma is a chem-e-car with 31 cm x 21 cm dimension powered by decomposition reaction of hydrogen peroxide 10% inside the vessel catalyzed by KI. The car has good corrosion resistance and is safe in the operation due to its safety valve used to maintain the pressure inside the vessel which is made from stainless steel 304. With no stopping mechanism, the car will stop when the oxygen gas in the vessel runs out. Compressed air engine is a type of engine that combines the principles of diesel engine and stirling engine using high pressure air with a high compression ratio so that it needs no spark plugs as a detonation process. The cycle of the piston in the engine uses 2 strokes, the intake valve is regulated by a push rod with a camshaft mechanism and an exhaust hole. The material used in manufacturing is PLA through the 3D printing method.
MORE CHEM E CARS